Message from the CHair
|
Dear Conference Attendees,
Welcome to the 26th Annual American Association of Pharmaceutical Scientists Northeast Regional Discussion Group (AAPS-NERDG) Meeting. This year we are hosting our conference in Groton-Mystic area which provides a great weekend getaway for those who like to explore quaint coastal towns. |
This year we have a lineup of impressive speakers and presenters from industry and academia alike. The conference features two keynote presentations. The morning keynote will be delivered by Dr. John Rogers, Professor and Director of Querrey-Simpson Institute of Bioelectronics at Northwestern University. Dr. Rogers’ research includes fundamental and applied aspects of nano and molecular scale fabrication as well as materials and patterning techniques for unusual electronic and photonic devices, with an emphasis on bio-integrated and bio-inspired systems. He will be sharing his vision about Biosensor’s role in pharmaceutical development. The afternoon keynote will be presented by Mr. Martin Warman, Director of Martin Warman Consultancy Limited, UK. Mr. Warman is an industry veteran with an impressive track record working in the fields of innovative engineering solutions, process analytical technology (PAT), and tech transfer to name a few. Mr. Warman will discuss about Transformational manufacturing that will allow us to make low-cost personalized products (combinations) via his broad career journey experiences.
After the morning keynote there will be three sessions of Short Topic Presentations (STP) with talks on a range of pharmaceutical research areas. Previous year’s attendees’ suggestions were very well received, and this will be our first year introducing awards for the best STP presenters from each category. Six students will present on their research to compete for the Academic Research Award (ARA). Winners from both sessions will be announced in the Awards Ceremony later in the day.
Before and after lunch, there are equally exciting scientific presentations by Pion, Inc., Simulations Plus and SOTAX followed by an exclusive Poster Session hour. All attendees will have an opportunity to interact with poster presenters and exchange scientific ideas. Four Round Table Discussion sessions will follow, which will include in-depth presentations by our panelists on emerging topics in pharmaceutical development. After our second keynote, our conference will conclude with an Awards Ceremony followed by a Networking Reception.
There will be a vendor exhibition throughout the day. Participants that visit all vendor booths will have a chance to win a raffle prize. We appreciate all our sponsors who support this event, so please take some time to visit their exhibition tables and listen to their technical talks. Last but not the least, thank you for the incredible group of volunteers who have tirelessly worked to organize this year’s conference. Putting together an event of national recognition requires a lot of commitment, planning, and flexibility. This meeting wouldn’t be possible without their hard work and dedication to AAPS-NERDG.
Please enjoy the conference!
Sincerely,
Anand Gupta, PhD
AAPS-NERDG Chair 2023-2024
Investigator-GSK
After the morning keynote there will be three sessions of Short Topic Presentations (STP) with talks on a range of pharmaceutical research areas. Previous year’s attendees’ suggestions were very well received, and this will be our first year introducing awards for the best STP presenters from each category. Six students will present on their research to compete for the Academic Research Award (ARA). Winners from both sessions will be announced in the Awards Ceremony later in the day.
Before and after lunch, there are equally exciting scientific presentations by Pion, Inc., Simulations Plus and SOTAX followed by an exclusive Poster Session hour. All attendees will have an opportunity to interact with poster presenters and exchange scientific ideas. Four Round Table Discussion sessions will follow, which will include in-depth presentations by our panelists on emerging topics in pharmaceutical development. After our second keynote, our conference will conclude with an Awards Ceremony followed by a Networking Reception.
There will be a vendor exhibition throughout the day. Participants that visit all vendor booths will have a chance to win a raffle prize. We appreciate all our sponsors who support this event, so please take some time to visit their exhibition tables and listen to their technical talks. Last but not the least, thank you for the incredible group of volunteers who have tirelessly worked to organize this year’s conference. Putting together an event of national recognition requires a lot of commitment, planning, and flexibility. This meeting wouldn’t be possible without their hard work and dedication to AAPS-NERDG.
Please enjoy the conference!
Sincerely,
Anand Gupta, PhD
AAPS-NERDG Chair 2023-2024
Investigator-GSK
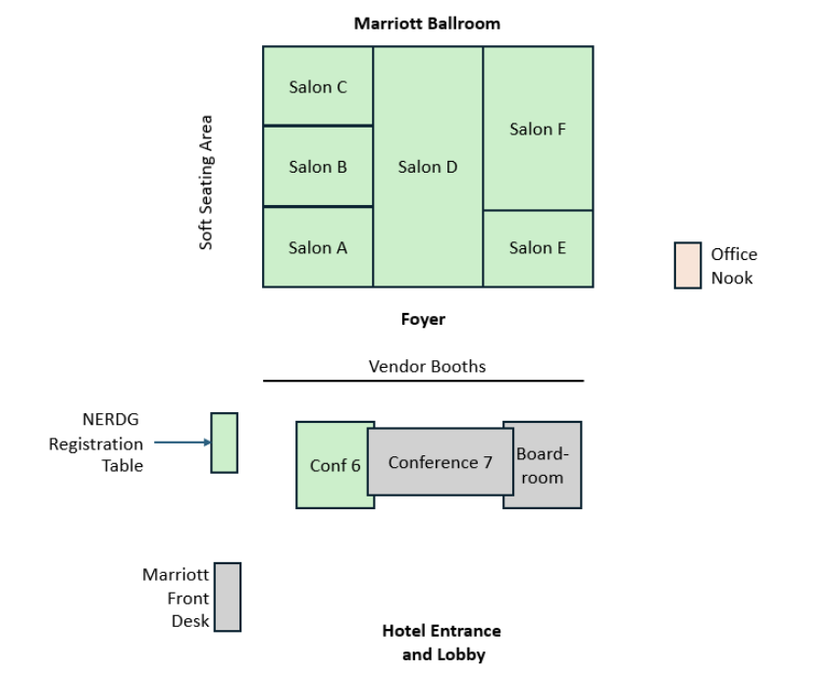
Program schedule
Time |
Program |
Location |
6:30 – 8:00 am |
Vendor Setup |
Marriott Ballroom Foyer |
7:00 – 8:00 am |
Poster Setup |
Marriott Salon D |
7:30 – 8:30 am |
Registration and Breakfast |
NERDG Registration Table and Marriott Ballroom Foyer |
8:30 – 8:45 am |
Introduction |
Marriott Salon E and F |
8:45 – 9:30 am |
Keynote Presentation 1 Title: Biosensor’s role in pharmaceutical development Speaker: John Rogers, Director, Simpson Institute for Bioelectronics |
Marriott Salon E and F |
9:30 – 9:45 am |
Vendor Presentation Pion |
Marriott Salon E and F |
9:45 – 10:00 am |
Break |
Marriott Ballroom Foyer |
10:00 am – 12:00 pm |
Academic Research Award Presentations Short Topic Presentations Session 1: Materials Science and Engineering Session 2: Drug Delivery and Packaging Solutions Session 3: Small Molecule Drug Product Development and Analysis |
Conference Room 6 Marriott Salon C Marriott Salon B Marriott Salon A |
12:00 – 12:45 pm |
Lunch |
Marriott Salon E and F |
12:45 – 1:15 pm |
Vendor Presentations Simulations Plus SOTAX |
Marriott Salon E and F |
1:15 – 2:15 pm |
Vendor Exhibits and Poster Presentations |
Marriot Foyer and Salon D Coffee and snacks available in foyer |
2:15 – 3:45 pm |
Round Table Presentations Simulations in Drug Development Evolving Biologics Landscape Nitrosamine Risk and Control Strategies Excipients - Novel Manufacturing Methods |
Marriott Salon C Marriott Salon B Marriott Salon A Conference Room 6 |
3:45 – 4:30 pm |
Keynote Presentation 2 Title: Transformational manufacturing that will allow us to make low-cost personalized products (combinations) Speaker: Martin Warman, Warman Consultancy |
Marriott Salon E and F |
4:30 – 4:45 pm |
Awards and Meeting Close |
Marriott Salon E and F |
4:45 – 5:30 pm |
Networking Reception |
Marriott Ballroom Foyer |
Event Photography
Photographs will be taken throughout the day. Please note that the photographs taken at this event may appear on materials such as our website and LinkedIn page. If you would prefer not to be photographed, please let the photographer know.
Keynote speakers
Keynote Speaker 1: Biosensor’s role in pharmaceutical development
John Rogers
Director, Simpson Institute for Bioelectronics
John Rogers
Director, Simpson Institute for Bioelectronics
Bio:
Professor John A. Rogers began his career at Bell Laboratories as a Member of Technical Staff in the Condensed Matter Physics Research Department in 1997, and served as Director from the end of 2000 to 2002. He then spent thirteen years at the University of Illinois, as the Swanlund Chair Professor and Director of the Seitz Materials Research Laboratory. In 2016, he joined Northwestern University as the Simpson/Querrey Professor, where he is also Director of the Institute for Bioelectronics. He has co-authored nearly 900 papers and he is co-inventor on more than 100 patents. His research has been recognized by many awards, including a MacArthur Fellowship (2009), the Lemelson-MIT Prize (2011), the Smithsonian Award for American Ingenuity in the Physical Sciences (2013), the Benjamin Franklin Medal (2019), and a Guggenheim Fellowship (2021). He is a member of the National Academy of Engineering, the National Academy of Sciences, the National Academy of Medicine and the American Academy of Arts and Sciences.
Soft, Skin-Interfaced Electronic and Microfluidic Sensors in Medicine
Abstract:
The skin is mechanically soft and curved; modern electronic and microfluidic technologies are rigid and planar. Eliminating this profound mismatch in physical properties will create vast opportunities in man-made systems that can naturally integrate with the epidermis, for diagnostic, therapeutic or sensory function with important, unique capabilities relevant to fitness/wellness, sports performance, clinical healthcare and virtual reality environments. Over the last decade, a convergence of new concepts in materials science, mechanical engineering, electrical engineering and advanced manufacturing has led to the emergence of diverse, novel classes of 'biocompatible' electronic and microfluidic systems with skin-like physical properties. This talk describes the key ideas and presents some of the most recent device examples, including wireless, battery-free electronic 'tattoos', with applications in continuous monitoring of vital signs in maternal, neonatal and pediatric populations, including active deployments in the most advanced hospitals in the US and clinics in multiple countries in Africa, microfluidic platforms that can capture, manipulate and perform biomarker analysis on microliter volumes of sweat, with applications in cystic fibrosis and nutritional monitoring. Important examples of these technologies in digital clinical trial support will be highlighted.
Professor John A. Rogers began his career at Bell Laboratories as a Member of Technical Staff in the Condensed Matter Physics Research Department in 1997, and served as Director from the end of 2000 to 2002. He then spent thirteen years at the University of Illinois, as the Swanlund Chair Professor and Director of the Seitz Materials Research Laboratory. In 2016, he joined Northwestern University as the Simpson/Querrey Professor, where he is also Director of the Institute for Bioelectronics. He has co-authored nearly 900 papers and he is co-inventor on more than 100 patents. His research has been recognized by many awards, including a MacArthur Fellowship (2009), the Lemelson-MIT Prize (2011), the Smithsonian Award for American Ingenuity in the Physical Sciences (2013), the Benjamin Franklin Medal (2019), and a Guggenheim Fellowship (2021). He is a member of the National Academy of Engineering, the National Academy of Sciences, the National Academy of Medicine and the American Academy of Arts and Sciences.
Soft, Skin-Interfaced Electronic and Microfluidic Sensors in Medicine
Abstract:
The skin is mechanically soft and curved; modern electronic and microfluidic technologies are rigid and planar. Eliminating this profound mismatch in physical properties will create vast opportunities in man-made systems that can naturally integrate with the epidermis, for diagnostic, therapeutic or sensory function with important, unique capabilities relevant to fitness/wellness, sports performance, clinical healthcare and virtual reality environments. Over the last decade, a convergence of new concepts in materials science, mechanical engineering, electrical engineering and advanced manufacturing has led to the emergence of diverse, novel classes of 'biocompatible' electronic and microfluidic systems with skin-like physical properties. This talk describes the key ideas and presents some of the most recent device examples, including wireless, battery-free electronic 'tattoos', with applications in continuous monitoring of vital signs in maternal, neonatal and pediatric populations, including active deployments in the most advanced hospitals in the US and clinics in multiple countries in Africa, microfluidic platforms that can capture, manipulate and perform biomarker analysis on microliter volumes of sweat, with applications in cystic fibrosis and nutritional monitoring. Important examples of these technologies in digital clinical trial support will be highlighted.
Keynote Speaker 2: Transformational manufacturing that will allow us to make low-cost personalized products (combinations)
Martin Warman
Warman Consultancy
Martin Warman
Warman Consultancy
Bio:
Martin is Director of Martin Warman Consultancy Ltd, providing consultancy services to many of the largest pharma companies. Previously he spent 7 years at Vertex, developing their continuous manufacturing (CM) platform used for the first approved CM products. He has over 25 years’ experience having previously spent 14 years at Pfizer Global manufacturing where he led the Global PAT Development Team. Martin is also an Associate Member of Centre for Process Analytics and Control Technology (CPACT), having recently stepped down as the Research Director for the UK’s Medicine Manufacturing Innovation Centre (MMIC).
Realizing the full potential of CM in the pharmaceutical innovator space
Abstract:
Although there is little doubt many companies are already realizing some benefits of switching from batch to continuous manufacturing (CM) paradigms, the first generation of deployments do not fully deliver on potential benefits in the low volume/innovator space.
Even second-generation deployments, which expand the use of continuous direct compression (CDC), leveraging the extended regime map (resulting from the more accommodating nature of CDC which regards to flowability, cohesivity and compression) do not fully realize potentials for ultra-rapid, product sparing development, as well as support flexible batch sizes, and potential for distributed and even point of care manufacturing.
Several large pharma companies, as well as industry collaborations (such as Medicine Manufacturing Innovation Centre, MMIC) or focused on a so-called third generation or mini-batch direct compression (m-bDC) approach. In m-bDC the overall line rate is continuous, with some process steps running continuously (for example tablet compression), but with dispensing, blending, discharge and material transfer occurring on a repeating small scale batchwise basis. Because these manufacturing steps are discrete plug flow, and because they are produced on an individual base, the process dynamics, at a given line rate, are identical between each mini-batch. It also means it is possible to demonstrate equivalence between individual and a series of mini-batches, both during development (to allow individual mini-batches to be representative of a batch), during commercial manufacturing (the batch size is simply the number of equivalent mini-batches), but also between 'mirror sites' running equivalent processes.
This presentation will chart the rapid progress towards m-bDC in the innovator space, as well as describe the mode of operation, the simplified control strategy required, unique opportunities for PPQ and on-going CPV as well as future 'pod-wise' manufacturing.
Martin is Director of Martin Warman Consultancy Ltd, providing consultancy services to many of the largest pharma companies. Previously he spent 7 years at Vertex, developing their continuous manufacturing (CM) platform used for the first approved CM products. He has over 25 years’ experience having previously spent 14 years at Pfizer Global manufacturing where he led the Global PAT Development Team. Martin is also an Associate Member of Centre for Process Analytics and Control Technology (CPACT), having recently stepped down as the Research Director for the UK’s Medicine Manufacturing Innovation Centre (MMIC).
Realizing the full potential of CM in the pharmaceutical innovator space
Abstract:
Although there is little doubt many companies are already realizing some benefits of switching from batch to continuous manufacturing (CM) paradigms, the first generation of deployments do not fully deliver on potential benefits in the low volume/innovator space.
Even second-generation deployments, which expand the use of continuous direct compression (CDC), leveraging the extended regime map (resulting from the more accommodating nature of CDC which regards to flowability, cohesivity and compression) do not fully realize potentials for ultra-rapid, product sparing development, as well as support flexible batch sizes, and potential for distributed and even point of care manufacturing.
Several large pharma companies, as well as industry collaborations (such as Medicine Manufacturing Innovation Centre, MMIC) or focused on a so-called third generation or mini-batch direct compression (m-bDC) approach. In m-bDC the overall line rate is continuous, with some process steps running continuously (for example tablet compression), but with dispensing, blending, discharge and material transfer occurring on a repeating small scale batchwise basis. Because these manufacturing steps are discrete plug flow, and because they are produced on an individual base, the process dynamics, at a given line rate, are identical between each mini-batch. It also means it is possible to demonstrate equivalence between individual and a series of mini-batches, both during development (to allow individual mini-batches to be representative of a batch), during commercial manufacturing (the batch size is simply the number of equivalent mini-batches), but also between 'mirror sites' running equivalent processes.
This presentation will chart the rapid progress towards m-bDC in the innovator space, as well as describe the mode of operation, the simplified control strategy required, unique opportunities for PPQ and on-going CPV as well as future 'pod-wise' manufacturing.
Short Topic Presentations (STP)
Session 1: Material Science and Engineering
Location: Marriott Salon C
Moderator: Jayshil Bhatt
Location: Marriott Salon C
Moderator: Jayshil Bhatt
Time |
Title |
Presenting Author |
Affiliation |
10:00 - 10:30 am |
Kevin DeBoyace |
Pfizer |
|
10:30 - 11:00 am |
Wesley Clark |
Pfizer |
|
11:00 - 11:30 am |
Maryam S. Sadeghi |
Pfizer |
|
11:30 am - 12:00 pm |
Vishvesh Raje |
St. John's University |
Session 2: Drug Delivery and Packaging Solutions
Location: Marriott Salon B
Moderator: Heather Frericks Schmidt
Location: Marriott Salon B
Moderator: Heather Frericks Schmidt
Time |
Title |
Presenting Author |
Affiliation |
10:00 - 10:30 am |
Eben Frenzel |
Pfizer |
|
10:30 - 11:00 am |
Ankit Rochani |
St. John Fisher University |
|
11:00 - 11:30 am |
Vikas Kumar |
University of Connecticut |
|
11:30 am - 12:00 pm |
Suman Pal |
University of Connecticut |
Session 3: Small Molecule Drug Product Development and Analysis
Location: Marriott Salon A
Moderator: Dimple Modi
Location: Marriott Salon A
Moderator: Dimple Modi
Time |
Title |
Presenting Author |
Affiliation |
10:00 - 10:30 am |
Owais Khalid |
Cornell University |
|
10:30 - 11:00 am |
Debbie Wanapun |
Pfizer |
|
11:00 - 11:30 am |
Radha Kulkarni |
University of Connecticut |
|
11:30 am - 12:00 pm |
Andrew G. Clark |
DigiM Solution |
Academic Research Award (ARA)
Location: Conference Room 6
Moderator: Snehal Shukla
Moderator: Snehal Shukla
Time |
Title |
Presenting Author |
Affiliation |
10:00 - 10:20 am |
Parasharamulu Kommarajula |
St. John's University |
|
10:20 - 10:40 am |
Mounika Pathuri |
University of Connecticut |
|
10:40 - 11:00 am |
Mural Quadros |
St. John's University |
|
11:00 - 11:20 am |
Nilesh Malavia |
University of Connecticut |
|
11:20 - 11:40 am |
Alec M. Diorio |
St. John's University |
|
11:40 am - 12:00 pm |
Venkatraman Nagarajan |
University of Connecticut |
Regular poster presentation (RPP)
Poster no. |
Title |
Presenting Author |
Affiliation |
1 |
Heather Frericks Schmidt |
Pfizer |
|
2 |
Marialuisa Berghela |
Albany College of Pharmacy and Health Sciences |
|
3 |
Michael Bielak and Tyler McDonald |
FreeThink Technologies, Inc. |
|
4 |
Mufaddal H Kathawala |
St. John's University |
|
5 |
Srinidi Mohan |
University of New England |
|
6 |
Arantxa Roach |
The City College of New York |
|
7 |
Rahul Haware |
Natoli Scientific |
|
8 |
Domick Lomonaco |
Albany College of Pharmacy and Health Sciences |
|
9 |
Arnaout |
City College of New York |
|
10 |
Sravani Reddy |
St. John's University |
|
11 |
Dana Gates |
Pfizer |
|
12 |
Shubham Sharma |
Pfizer |
|
13 |
Stacey Marden |
AstraZeneca |
|
14 |
Hansol Lee |
Pfizer |
|
15 |
Zhenzi Hong |
Albany College of Pharmacy and Health Sciences |
|
16 |
Jianyan Wang |
AstraZeneca |
|
17 |
Pratyusha Ghosh |
The City College of New York |
|
18 |
Amit Chandra Das |
Duquesne University |
|
19 |
Michael Safo Oduro |
Pfizer |
|
20 |
Kellyn M. P. Zagaja |
Pfizer |
|
21 |
Linnea Budge and Joshua Dalo |
FreeThink Technologies |
|
22 |
Joseph DeGennaro |
FreeThink Technologies |
|
23 |
Anastasiia Vasylaki |
The City College of New York |
|
24 |
S Cook |
AstraZeneca |
|
25 |
Alisha Patel |
MCPHS University |
|
26 |
Neel Akshay Shah |
MCPHS University |
|
27 |
Trushenkumar Shah |
University of Connecticut |
|
28 |
Leila Sharifi |
University of Connecticut |
Round table sessions
Simulations in Drug Development – Marriott Salon C
Multi-scale Computer Modeling for Advanced Pharmaceutical Manufacturing
Bodhi Chaudhuri, Ph.D.
Professor, Department of Pharmaceutical Sciences and Chemical Engineering
University of Connecticut
Abstract:
Drug development and manufacturing are one of the significant processes in the pharmaceutical industry. Various computational methods have been dramatically reducing the time and cost of drug discovery and development. Improved algorithms, overgrowing powerful computing architectures and the accelerating growth of rich data sets and AI/ML are driving advances in multiscale modelling methods capable of bridging chemical and biological complexity from the atom to the manufacturing scale. In this talk, the roles of physics based multiscale modeling and AI/ML in the areas of continuous manufacturing of injectables will be discussed. These research efforts yielded interesting results that provided insight into the effects of various material properties and operating conditions on the success of pharmaceutical processes.
Multi-scale Computer Modeling for Advanced Pharmaceutical Manufacturing
Bodhi Chaudhuri, Ph.D.
Professor, Department of Pharmaceutical Sciences and Chemical Engineering
University of Connecticut
Abstract:
Drug development and manufacturing are one of the significant processes in the pharmaceutical industry. Various computational methods have been dramatically reducing the time and cost of drug discovery and development. Improved algorithms, overgrowing powerful computing architectures and the accelerating growth of rich data sets and AI/ML are driving advances in multiscale modelling methods capable of bridging chemical and biological complexity from the atom to the manufacturing scale. In this talk, the roles of physics based multiscale modeling and AI/ML in the areas of continuous manufacturing of injectables will be discussed. These research efforts yielded interesting results that provided insight into the effects of various material properties and operating conditions on the success of pharmaceutical processes.
|
Bio: Bodhi Chaudhuri is a Professor of the Department of Pharmaceutical Sciences and Chemical Engineering at University of Connecticut. He held visiting professor positions in University of Copenhagen, National University of Singapore, and Monash University, Australia. He has published over 100 peer reviewed journal papers, book chapters, and conference proceedings. He has delivered more than 75 invited talks in academia, federal laboratories, and industries along with more than 200 conference presentations. He serves on the program committees of international conferences, and acts as an editorial board member of more than 10 journals. |
He currently serves as the elected executive board member of the Powder Technology Forum of AIChE and was the elected Chair of the Advanced Manufacturing Focus group of NIPTE. He has received several prestigious awards from PhRMA Foundation, FDA, NIPTE, and Department of Education in India. Congressman Joe Courtney applauded the research efforts of Chaudhuri-Group in United States Congressional Report in 2011. He is an elected member of Connecticut Academy of Science and Engineering (CASE) and the co-founder of QUASIM.
Smart Manufacturing of Downstream Pharmaceutical Drug Products
Rohit Ramachandran, Ph.D.
Professor, Dept. of Chemical and Biochemical Engineering,
Rutgers University
Abstract:
The global pharmaceuticals market is a trillion-dollar industry, but the pharmaceutical sector lags in manufacturing innovation and automation which limits its potential to maximize product quality and energy efficiency. The implementation of a smart manufacturing (SM) platform can help enterprises create business models, which can be adapted for the energy-demanding manufacturing process to reduce energy consumption while ensuring product quality, thus ensuring more sustainable process operations. In the developed smart manufacturing platform, process data of three unit-operations (granulation, drying and milling) involved in the downstream manufacturing of solid based dosage forms such as tablets/capsules, are collected using a combination of a manufacturing execution system (MES) and an electronic laboratory notebook (ELN). The collected data are then transferred to a central cloud data lake, from which they can be imported into integrated process models for predicting product quality and energy efficiency. This data is also used to perform techno-economic analysis (TEA) and process optimization to minimize energy and maximize tablet therapeutic efficiency. The process models are developed using first principle-based techniques which can accurately predict the critical quality attributes of the final product. The energy efficiency of the process is calculated using statistical models relating the power consumption and the process parameters. Using the developed process models and process data from the SM platform, the proposed framework can quantitatively assess the energy and cost savings as the process is being optimized. Utilizing the integrated SM platform, the pharmaceutical manufacturing process is optimized to maintain product quality and achieve an energy savings of over 20%.
Rohit Ramachandran, Ph.D.
Professor, Dept. of Chemical and Biochemical Engineering,
Rutgers University
Abstract:
The global pharmaceuticals market is a trillion-dollar industry, but the pharmaceutical sector lags in manufacturing innovation and automation which limits its potential to maximize product quality and energy efficiency. The implementation of a smart manufacturing (SM) platform can help enterprises create business models, which can be adapted for the energy-demanding manufacturing process to reduce energy consumption while ensuring product quality, thus ensuring more sustainable process operations. In the developed smart manufacturing platform, process data of three unit-operations (granulation, drying and milling) involved in the downstream manufacturing of solid based dosage forms such as tablets/capsules, are collected using a combination of a manufacturing execution system (MES) and an electronic laboratory notebook (ELN). The collected data are then transferred to a central cloud data lake, from which they can be imported into integrated process models for predicting product quality and energy efficiency. This data is also used to perform techno-economic analysis (TEA) and process optimization to minimize energy and maximize tablet therapeutic efficiency. The process models are developed using first principle-based techniques which can accurately predict the critical quality attributes of the final product. The energy efficiency of the process is calculated using statistical models relating the power consumption and the process parameters. Using the developed process models and process data from the SM platform, the proposed framework can quantitatively assess the energy and cost savings as the process is being optimized. Utilizing the integrated SM platform, the pharmaceutical manufacturing process is optimized to maintain product quality and achieve an energy savings of over 20%.
|
Bio:
Dr. Rohit Ramachandran is currently full Professor at the Dept. of Chemical & Biochemical Engineering at Rutgers University, NJ, USA. His research interests are at the interface of Process Systems Engineering and Particle/Chemical Technology with applications in Pharmaceutical and Chemical Processes. He has published over 130 journal papers and 8 book chapters in these areas and has presented his work at numerous conferences and invited seminars. His research work has been cited more than 5500 times with a H-index of 44. He has received several awards such as the National Science Foundation (NSF) CAREER award, the National Institute of Technology and Education (NIPTE) Young Investigator award, the American Institute of Chemical Engineering (AIChE) Quality-by-Design in Drug product manufacturing award, and the Rutgers Chancellor’s Scholar and Rutgers Board of Trustees awards. |
Challenges in Implementing Practical Deep Learning-Based Image Analysis Workflows for
PAT Monitoring of Crystallization
Helen Yao, PhD
Principal Investigator, GlaxoSmithKline
Abstract:
Image-based process analytical technology (PAT) is frequently used to monitor crystallizations in real-time. The ability to extract quantitative particle metrics, such as particle size distribution and chord length, from images is extremely valuable, but to achieve this, robust image analysis algorithms are required. Here, we explore the practical challenges of implementing a fully automated image analysis workflow for images obtained by an EasyViewer 100 probe during crystallization. A deep learning-based image segmentation algorithm with UNet architecture was built to generate crystal predictions to calculate particle size distributions from images. The algorithm was able to accurately identify crystals with the same habit as those from the training dataset. However, the algorithm did not perform reliably for images with crystals of different crystal habits and for images containing various artifacts, even if these artifacts are included as part of the training dataset. We address possible solutions for model improvement and broader roadblocks to implementing such a workflow to a real industrial process.
PAT Monitoring of Crystallization
Helen Yao, PhD
Principal Investigator, GlaxoSmithKline
Abstract:
Image-based process analytical technology (PAT) is frequently used to monitor crystallizations in real-time. The ability to extract quantitative particle metrics, such as particle size distribution and chord length, from images is extremely valuable, but to achieve this, robust image analysis algorithms are required. Here, we explore the practical challenges of implementing a fully automated image analysis workflow for images obtained by an EasyViewer 100 probe during crystallization. A deep learning-based image segmentation algorithm with UNet architecture was built to generate crystal predictions to calculate particle size distributions from images. The algorithm was able to accurately identify crystals with the same habit as those from the training dataset. However, the algorithm did not perform reliably for images with crystals of different crystal habits and for images containing various artifacts, even if these artifacts are included as part of the training dataset. We address possible solutions for model improvement and broader roadblocks to implementing such a workflow to a real industrial process.
|
Bio: Dr. Helen Yao is a Principal Investigator at GSK in the Process Engineering and Analytics (PE&A) team, where she develops computational models to support small and large molecule drug substance and drug product development. Dr. Yao completed her B.S.E. in Chemical and Biological Engineering in 2015 at Princeton University. In 2020, she received her Ph.D. in Chemical Engineering at Massachusetts Institute of Technology in the lab of Prof. Bradley Olsen, where she studied self-assembly of protein-polymer bioconjugates using scattering and molecular dynamics simulation. At GSK, Dr. Yao’s research focus is mechanistic, data-driven, and hybrid process model development and deployment to accelerate drug delivery to patients. In addition to her scientific work, she is passionate about STEM outreach and has organized events for the PE&A team at local Philly high schools. |
Evolving Biologics Landscape – Marriott Salon B
Continuous Manufacturing Platform for Nanoparticle-Based Complex Parenteral Therapeutics
Diane J. Burgess, Ph.D.
Distinguished Professor, Pfizer Distinguished Chair in Pharmaceutical Technology
University of Connecticut.
Abstract:
Following the Covid 19 pandemic, it has become even more apparent that advanced manufacturing approaches that can achieve speed to market of high-quality injectable products at high throughput and low cost, with a small footprint that allows ease of deployment to locations of need for rapid on demand manufacturing. This presentation will focus on the development of a novel continuous manufacturing platform for complex parenterals, such as the Covid 19 mRNA vaccines. Currently there are 15 US FDA approved products produced via continuous manufacturing. However, all of these products are in the solid oral space. The benefits associated with continuous manufacturing include: increased quality through online process analytical technology (PAT) to achieve rapid production of high-quality products, increased throughput, small foot print, no scale up issues, and reduced cost.
Our laboratory has developed a novel continuous manufacturing platform for complex parenteral dosage forms which allows precise control over particle size and can also ensure monodisperse particles. This platform is fully equipped with PAT to ensure all aspects of product quality. An overview of this manufacturing platform will be presented. The platform is based on co-flow technology and employs the formation of a turbulent jet at the site where the two flows mix, promoting vesicle formation. Case studies on different therapeutics (liposomes, mRNA lipid nanoparticles (LNPs) and polymeric micelles) prepared using this technology will be discussed as well as the utilization of this technology for quality standards preparation for complex parenterals.
Continuous Manufacturing Platform for Nanoparticle-Based Complex Parenteral Therapeutics
Diane J. Burgess, Ph.D.
Distinguished Professor, Pfizer Distinguished Chair in Pharmaceutical Technology
University of Connecticut.
Abstract:
Following the Covid 19 pandemic, it has become even more apparent that advanced manufacturing approaches that can achieve speed to market of high-quality injectable products at high throughput and low cost, with a small footprint that allows ease of deployment to locations of need for rapid on demand manufacturing. This presentation will focus on the development of a novel continuous manufacturing platform for complex parenterals, such as the Covid 19 mRNA vaccines. Currently there are 15 US FDA approved products produced via continuous manufacturing. However, all of these products are in the solid oral space. The benefits associated with continuous manufacturing include: increased quality through online process analytical technology (PAT) to achieve rapid production of high-quality products, increased throughput, small foot print, no scale up issues, and reduced cost.
Our laboratory has developed a novel continuous manufacturing platform for complex parenteral dosage forms which allows precise control over particle size and can also ensure monodisperse particles. This platform is fully equipped with PAT to ensure all aspects of product quality. An overview of this manufacturing platform will be presented. The platform is based on co-flow technology and employs the formation of a turbulent jet at the site where the two flows mix, promoting vesicle formation. Case studies on different therapeutics (liposomes, mRNA lipid nanoparticles (LNPs) and polymeric micelles) prepared using this technology will be discussed as well as the utilization of this technology for quality standards preparation for complex parenterals.
|
Bio: Dr. Burgess received her B.Sc. degree in Pharmacy from the University of Strathclyde, U.K. (1979) and her Ph.D. in Pharmaceutics from the University of London, U.K. (1984). She was a postdoctoral fellow at the Universities of Nottingham, U.K. (1984-1985) and North Carolina (1985). Dr. Burgess joined the faculty at the University of Illinois at Chicago in 1986 as Assistant and then Associate Professor and moved to the University of Connecticut in 1993. She was promoted to Professor in 1999, appointed Board of Trustees Distinguished Professor of Pharmaceutics in 2009, before becoming the Pfizer Distinguished Chair in Pharmaceutical Technology in 2018. In 2019, Dr. Burgess founded DIANT Pharma, which develops proprietary continuous manufacturing technology for nanoparticles with a wide array of applications throughout multiple industries, such as lipid nanoparticles and liposomes for pharmaceutical formulations. |
Controlled release and enhanced stabilization of nucleic acid therapeutics using DNA-surfactant conjugates
Jessica Rouge, PhD
Assistant Professor, Department of Chemistry, University of Connecticut
Abstract:
Our laboratory has worked to develop formulations for delivering therapeutic nucleic acids that can stabilize, target, and control the release of DNA and RNA molecules into cells. A major obstacle in the field of nucleic acid therapeutics is the ability to successfully deliver these biomolecules to the cytosol. Overcoming the lipid membrane barriers of cells and endosomes is the major bottleneck to this process. We have thus designed the chemistry of our formulations to outfit nucleic acids with the ability to destabilize membrane barriers while also presenting targeting ligands and degradation mechanisms that can help control the trajectory of nucleic acid cargo once inside a cell. In this talk I will summarize our latest advancements in delivering important nucleic acid cargo, both short therapeutic oligonucleotides and larger molecules such as mRNA.
Jessica Rouge, PhD
Assistant Professor, Department of Chemistry, University of Connecticut
Abstract:
Our laboratory has worked to develop formulations for delivering therapeutic nucleic acids that can stabilize, target, and control the release of DNA and RNA molecules into cells. A major obstacle in the field of nucleic acid therapeutics is the ability to successfully deliver these biomolecules to the cytosol. Overcoming the lipid membrane barriers of cells and endosomes is the major bottleneck to this process. We have thus designed the chemistry of our formulations to outfit nucleic acids with the ability to destabilize membrane barriers while also presenting targeting ligands and degradation mechanisms that can help control the trajectory of nucleic acid cargo once inside a cell. In this talk I will summarize our latest advancements in delivering important nucleic acid cargo, both short therapeutic oligonucleotides and larger molecules such as mRNA.
|
Bio: Jessica received her a Ph.D. in Chemistry from Prof. Bruce E. Eaton’s laboratory at the University of Colorado in 2012. There she studied chemically modified RNA sequences that bind inorganic nanomaterials isolated via in vitro selections using SELEX, resulting in aptamers for a variety of applications. After completing her Ph.D., Jessica began her postdoctoral studies in 2013 in the laboratory of Prof. Chad A. Mirkin at Northwestern University where she was supported by a PhRMA Foundation postdoctoral fellowship in Pharmaceutics. In the Mirkin Laboratory she applied the skills she learned for interfacing RNA and inorganic materials for developing enzymatic assembly approaches useful for synthesizing RNA gold nanoparticle conjugates that could regulate intracellular gene expression. In 2015, Jessica joined the faculty of the University of Connecticut, where she is currently an Associate Professor in the Department of Chemistry. |
She is the recipient of an NSF CAREER award and a Maximizing Investigators Research Award (MIRA) for early-stage investigators from the NIH. At UConn she combines the chemical diversity of nucleic acids with the synthesis of nanoscale materials for a wide variety of applications in biological systems. A major current focus of her group is on developing new chemistries and materials for the more effective delivery of therapeutic nucleic acids, including her most recent formulation that has shown success in applications related to airway diseases.
New Manufacturing Strategies to Accelerate RNA-LNP Development
Jason Coleman, PhD
Precision NanoSystems
Abstract:
RNA can be designed to silence, express and edit specific genes, providing a flexible and powerful approach to treating diseases. COVID-19 mRNA vaccines, while an extraordinary accomplishment, have revealed that rapid development and scale-up, as well as access to innovative technologies, are needed to keep up with emerging variants and to usher in a new wave of genomic medicines to address other areas of unmet medical need. In this presentation, I will share insights on cutting-edge technologies accelerating the development of RNA-lipid nanoparticle-based medicines from discovery to commercialization.
Jason Coleman, PhD
Precision NanoSystems
Abstract:
RNA can be designed to silence, express and edit specific genes, providing a flexible and powerful approach to treating diseases. COVID-19 mRNA vaccines, while an extraordinary accomplishment, have revealed that rapid development and scale-up, as well as access to innovative technologies, are needed to keep up with emerging variants and to usher in a new wave of genomic medicines to address other areas of unmet medical need. In this presentation, I will share insights on cutting-edge technologies accelerating the development of RNA-lipid nanoparticle-based medicines from discovery to commercialization.
|
Bio:
Jason is currently the Global Lead Field Application Scientist (FAS) leading a team of FASs who support clients as they integrate, develop and scale-up their nanoparticle-based formulations on PNI’s NanoAssemblr™ novel mixing technology. Jason joined PNI in 2018 as a Field Application Scientist where he focused on client education and pre-clinical nanoparticle formulation development. Jason’s role then shifted to the Lead Clinical FAS, dedicated to further supporting clients as they scaled-up their nanoparticle-based formulations on the path to the clinic. Prior to joining PNI, Jason worked as an Associate Director to lead a team focused on the integration of a nanoparticle-based platform for the delivery of small molecules for bioavailability enhancement. |
He was the lead scientist on the development of a prostate cancer drug that was commercially approved on this platform. Jason also worked for several years as a Process and Validation Engineer in the Pharma and Biotech industries. Overall, Jason has over 15 years of experience in the pharmaceutical and biotech industries and has specialized in both nanoparticle platform drug development and process scale-up. Jason received his Ph.D. from Drexel University in Chemical and Biological Engineering. His research was based on the development of a biodegradable polymeric nano- and micro- particle delivery system for the sustained delivery of therapeutic proteins.
Nitrosamine Risks and Control Strategies – Marriott Salon A
Nitrosamine Risk Assessment in Pharmaceuticals: Managing N-Nitrosamines and their Risk Assessment within GSK R&D
Alan Shaffer
Senior Scientist, GSK Medicine Development & Supply, Collegeville, PA
Abstract:
Pharma develop small molecule products to deliver benefit to the patient. Impurities present in drug substances and/or drug products do not add any benefit to the patient, and in some cases could cause harm. The N-nitrosamine chemical class is in the “cohort of concern” within the ICH M7 guideline as some of these compounds have been shown to be highly potent mutagenic carcinogens in rodent studies. Given their marked carcinogenic potency, GSK continues to adopt the dynamic and evolving regulatory position with N-nitrosamines in combination with the principles outlined in ICH M7 for the assessment and control of mutagenic impurities as appropriate. The Mutagenic Impurity Risk Assessment is a cross-functional review of the manufacturing process used to prepare clinical grade drug substance and drug product for human clinical trials and for subsequent commercial production. The aim of this risk assessment process is to identify and assess impurities formed during the synthesis of drug substances, as well as identify and assess impurities and degradants that may be present in drug substances and products and that could be potential mutagenic carcinogens. This presentation will focus on the assessment of products for one particular class of mutagenic impurities which reside within the ICH M7 “cohort of concern”.
Nitrosamine Risk Assessment in Pharmaceuticals: Managing N-Nitrosamines and their Risk Assessment within GSK R&D
Alan Shaffer
Senior Scientist, GSK Medicine Development & Supply, Collegeville, PA
Abstract:
Pharma develop small molecule products to deliver benefit to the patient. Impurities present in drug substances and/or drug products do not add any benefit to the patient, and in some cases could cause harm. The N-nitrosamine chemical class is in the “cohort of concern” within the ICH M7 guideline as some of these compounds have been shown to be highly potent mutagenic carcinogens in rodent studies. Given their marked carcinogenic potency, GSK continues to adopt the dynamic and evolving regulatory position with N-nitrosamines in combination with the principles outlined in ICH M7 for the assessment and control of mutagenic impurities as appropriate. The Mutagenic Impurity Risk Assessment is a cross-functional review of the manufacturing process used to prepare clinical grade drug substance and drug product for human clinical trials and for subsequent commercial production. The aim of this risk assessment process is to identify and assess impurities formed during the synthesis of drug substances, as well as identify and assess impurities and degradants that may be present in drug substances and products and that could be potential mutagenic carcinogens. This presentation will focus on the assessment of products for one particular class of mutagenic impurities which reside within the ICH M7 “cohort of concern”.
|
Bio:
Prior to joining GSK, Alan worked for several years as a Synthetic Organic Chemist at DOW Agrosciences working with insecticides, herbicides, and fungicides in both the Lead Generation and Lead Optimization spaces. In addition to his prior industrial experience, Alan worked in academia teaching Organic, Nursing, and General Chemistry courses, as well as research and academic laboratory director and program coordinator roles. Alan joined GSK in 2021 where he has functioned as a high performing drug substance development chemist as well as providing challenge within the genotoxic risk assessment, nitrosamine, and general impurities management space. |
Addressing Nitrosamines in Pharmaceuticals – USP Perspective and Activities
Edmond Biba
Senior Principal Scientist, U.S. Pharmacopeial Convention, Rockville, MD
Abstract:
The presentation will cover the USP’s immediate and continuous response since nitrosamines emergence as public health concern for pharmaceutical products and pharmacopeia perspective in addressing and mitigating the risk of nitrosamines. The USP perspective and response is comprised of a multiprong approach including: 1) development of documentary standards – general chapter <1469> Nitrosamine Impurities–; 2) Physical USP Nitrosamine Reference Standards associated with this chapter and other nitrosamine reference standards of interests for pharmaceutical industry; 3) Advocacy – raising public awareness by disseminating information and providing education using several mechanisms available to USP, including USP education courses and other USP provided training, creation and maintenance of Nitrosamine Exchange (a USP moderated online Community), on demand video training etc.; 4) Providing tools and solutions – Analytical Methods developed by USP and/or in collaboration with other organization and sharing them, and approaches on performing risk assessments. USP’s road ahead regarding nitrosamines will also be discussed.
Edmond Biba
Senior Principal Scientist, U.S. Pharmacopeial Convention, Rockville, MD
Abstract:
The presentation will cover the USP’s immediate and continuous response since nitrosamines emergence as public health concern for pharmaceutical products and pharmacopeia perspective in addressing and mitigating the risk of nitrosamines. The USP perspective and response is comprised of a multiprong approach including: 1) development of documentary standards – general chapter <1469> Nitrosamine Impurities–; 2) Physical USP Nitrosamine Reference Standards associated with this chapter and other nitrosamine reference standards of interests for pharmaceutical industry; 3) Advocacy – raising public awareness by disseminating information and providing education using several mechanisms available to USP, including USP education courses and other USP provided training, creation and maintenance of Nitrosamine Exchange (a USP moderated online Community), on demand video training etc.; 4) Providing tools and solutions – Analytical Methods developed by USP and/or in collaboration with other organization and sharing them, and approaches on performing risk assessments. USP’s road ahead regarding nitrosamines will also be discussed.
|
Bio: Dr. Edmond Biba is a Senior Principal Scientist in the General Chapters Department-Science Division at United States Pharmacopeial Convention. He is responsible for managing the development of several new General Chapters USP-NF as well as revisions of existing ones, including <1430.X> suite of chapters on Analytical Methodologies Based on Scattering Phenomena, <1469> Nitrosamine Impurities, <1776> Image Analysis of Pharmaceutical Systems, <467> Residual Solvents, <197> Spectroscopic Identification Tests, with the help of USP volunteers, Expert Committee/ Expert Panel members. Dr. Biba received his Ph.D. in Chemistry-Synthetic Organic Chemistry from the American University, Washington DC, and a B.Sc. in Chemical Engineering/Chemistry from University of Tirana, Tirana, Albania. |
Dr. Biba is member of Sigma Xi – The Scientific Research Honor Society, American Chemical Society, American Association of Pharmaceutical Scientists. He is USP’s representative to ICH’s Residual Solvents Q3C (R10) and Mutagenic Impurities M7 (R3) Working Groups, and one of the USP’s representative to the Product Quality Technical Committee (PQTC) of the Product Quality Research Institute (PQRI).
Setting Acceptable Intakes (AIs) for Complex Nitrosamines
Michelle Kenyon
Associated Research Fellow, Pfizer Research & Development, Groton, CT
Abstract:
Low levels of nitrosamines have been shown to form in some pharmaceutical products that contain amines that are vulnerable to nitrosation. Nitrosamines are a class of compounds that are in the cohort of concern, a group of potentially potent animal carcinogens. Therefore, they can require control to levels below the threshold of toxicological concern defined in ICHM7 for controlling mutagenic impurities. However, recent evaluations show that the carcinogenic potency of nitrosamines ranges 4 orders of magnitude, and many are not carcinogenic. Despite this, in the absence of carcinogenicity data on a specific nitrosamine, health authorities are often recommending limits based on AIs established for some of the most potent small molecular weight nitrosamines. As nitrosamines related to drugs (NDSRIs) typically do not have carcinogenicity data and are much more complex than the small molecular weight nitrosamines for which there is carcinogenic potency data, it is important to consider structural features that enhance or mitigate carcinogenic potency in deriving an AI. Additionally, as mutagenicity is a key molecular event required for a nitrosamine to be carcinogenic, consideration of mutagenicity data generated for an NDSRI can also aid in derivation of an AI. This presentation will discuss some methods for categorizing and deriving AIs for complex nitrosamines. Case studies presenting derivation of AIs based on various structure-based approaches, including the recent Carcinogenic Potency Categorization Approach published by various health authorities will be shared. These will be compared with AIs derived based on experimental mutagenicity data.
Michelle Kenyon
Associated Research Fellow, Pfizer Research & Development, Groton, CT
Abstract:
Low levels of nitrosamines have been shown to form in some pharmaceutical products that contain amines that are vulnerable to nitrosation. Nitrosamines are a class of compounds that are in the cohort of concern, a group of potentially potent animal carcinogens. Therefore, they can require control to levels below the threshold of toxicological concern defined in ICHM7 for controlling mutagenic impurities. However, recent evaluations show that the carcinogenic potency of nitrosamines ranges 4 orders of magnitude, and many are not carcinogenic. Despite this, in the absence of carcinogenicity data on a specific nitrosamine, health authorities are often recommending limits based on AIs established for some of the most potent small molecular weight nitrosamines. As nitrosamines related to drugs (NDSRIs) typically do not have carcinogenicity data and are much more complex than the small molecular weight nitrosamines for which there is carcinogenic potency data, it is important to consider structural features that enhance or mitigate carcinogenic potency in deriving an AI. Additionally, as mutagenicity is a key molecular event required for a nitrosamine to be carcinogenic, consideration of mutagenicity data generated for an NDSRI can also aid in derivation of an AI. This presentation will discuss some methods for categorizing and deriving AIs for complex nitrosamines. Case studies presenting derivation of AIs based on various structure-based approaches, including the recent Carcinogenic Potency Categorization Approach published by various health authorities will be shared. These will be compared with AIs derived based on experimental mutagenicity data.
|
Bio:
Michelle graduated from Quinnipiac College in 1993 with a BA in Biology. She joined the Genetic Toxicology group at Pfizer in 1993 and earned an MS in Biology from Brown University in 2000 while working at Pfizer. She has fulfilled functions encompassing lab technician, lab technical lead, Study Director and genetic toxicology and impurity subject matter expert. In 2021, Michelle joined the Toxicology Impurity Risk Management (TIRM) team at Pfizer in Drug Safety R&D (DSRD). TIRM is a network team delivering high-quality risk assessments and impurity consultation across the entire Pfizer portfolio. With a specialist knowledge of impurity management, Michelle represents Drug Safety and co-leads a cross discipline teams discussing safety and quality aspects of impurity control. |
Over the last 23 years, she has built expertise in mutagenic impurities leading Pfizer’s in silico assessment team and co-leading a cross functional team that establishes acceptable intakes (Ais) for nitrosamines in the portfolio. She has also developed expertise in non-mutagenic impurity, extractable and leachable (E&L), and contaminant risk assessments, contributing to the work of the TIRM for years before joining the team. Michelle’s expertise is recognised externally where she has worked collaboratively in cross industry groups to develop published AIs and permissible daily exposure limits (Bercu et al, 2018; Johnson et al, 2021; Dobo et al, 2022; Bercu et al, 2023) and has contributed to development and review of ICH M7 monographs for the Addendums to ICH M7 (R1) and (R2). She is also a strong contributor to the Lhasa AI/PDE collaboration where she develops and critically reviews monographs for both mutagenic and non-mutagenic impurities. Michelle is also involved in the International Consortium for Innovation and Quality (IQ) DruSafe Impurities workgroup and the European Federation of Pharmaceutical Industries and Associations (EFPIA) Impurities Management Workgroup where she has respectively been co-leading and leading subgroups that are developing manuscripts related to non-mutagenic impurity management.
Excipients – Novel Manufacturing Methods – Conference Room 6
Perfluorocarbons as Multifunctional Building Blocks in Biomaterials
Jelena M. Janjic, PhD “Dr. J”
Associate Professor of Pharmaceutics
Duquesne University School of Pharmacy, Pittsburgh, PA
Abstract:
Perfluorocarbons (PFCs) have been used in medicine for over four decades. Interest in PFCs increased exponentially following their first clinical applications as blood substitutes in the late 1980s. They are biologically and chemically inert materials with fluorine atoms replacing hydrogens on a linear, branched, or cyclic carbon backbone. The strength of the C-F bond gives PFCs unique physicochemical properties, such as the high capacity for dissolving gasses. This is why they are suitable oxygen carriers and are metabolically inert. PFCs also can form powerful surfactants when conjugated to lipids or PEGs. They are mostly formulated as colloidal dispersions (e.g., nanoemulsions) or can be mixed with polymers forming hydrogels and conjugated to fluorescent reporters, targeting agents, or biosensors. They are also tracers for 19F magnetic resonance imaging, a virtually background-free quantitative imaging technique for therapeutic immune cell tracking and imaging inflammation in cardiovascular diseases, cancer, organ rejection, and lung disease. In this talk, I will discuss the physicochemical properties of PFCs and provide examples of their use in biomaterials. I will also discuss the impact of quality-by-design on PFC development, first implemented by our laboratory, and propose the pathways to PFC clinical translation in drug delivery, imaging, and regenerative medicine.
Perfluorocarbons as Multifunctional Building Blocks in Biomaterials
Jelena M. Janjic, PhD “Dr. J”
Associate Professor of Pharmaceutics
Duquesne University School of Pharmacy, Pittsburgh, PA
Abstract:
Perfluorocarbons (PFCs) have been used in medicine for over four decades. Interest in PFCs increased exponentially following their first clinical applications as blood substitutes in the late 1980s. They are biologically and chemically inert materials with fluorine atoms replacing hydrogens on a linear, branched, or cyclic carbon backbone. The strength of the C-F bond gives PFCs unique physicochemical properties, such as the high capacity for dissolving gasses. This is why they are suitable oxygen carriers and are metabolically inert. PFCs also can form powerful surfactants when conjugated to lipids or PEGs. They are mostly formulated as colloidal dispersions (e.g., nanoemulsions) or can be mixed with polymers forming hydrogels and conjugated to fluorescent reporters, targeting agents, or biosensors. They are also tracers for 19F magnetic resonance imaging, a virtually background-free quantitative imaging technique for therapeutic immune cell tracking and imaging inflammation in cardiovascular diseases, cancer, organ rejection, and lung disease. In this talk, I will discuss the physicochemical properties of PFCs and provide examples of their use in biomaterials. I will also discuss the impact of quality-by-design on PFC development, first implemented by our laboratory, and propose the pathways to PFC clinical translation in drug delivery, imaging, and regenerative medicine.
|
Bio:
Jelena M. Janjic, PhD, also known as “Dr. J”, is a tenured Associate Professor of Pharmaceutics and Founder/Co-Director of the Chronic Pain Research Consortium (CPRC, www.duq.edu/pain [duq.edu]) at Duquesne University in Pittsburgh, USA. She received her pharmacy degree from Belgrade University. Yugoslavia in 1998 and her Ph.D. at the University of Pittsburgh School of Pharmacy in 2005. She completed her post-doctoral training at Scripps Florida (2006) and Carnegie Mellon University (2009). The primary focus of her research is implementing Quality by Design in manufacturing nanomedicines for imaging-supported drug delivery. |
In her 17-year-long career in nanotechnology, she has developed multiple imaging and drug delivery platforms and biomaterials, which resulted in 3 patents, more than 60 publications, and numerous invited presentations at national and international meetings. Her research interests lie at the intersection of immunology and neuroscience in the control of neuroinflammation in pain, trauma, and regenerative medicine. Specifically, her work has focused on nanomedicines, which simultaneously image and modulate immune cells for therapeutic intervention in trauma and post-surgical pain, pain following neuromusculoskeletal injuries, neuropathic and chronic inflammatory pain. She also works in organ transplantation and rejection control, regenerative medicine, and organ preservation. Her designs have been successfully validated in experimental small and large animal models of transplant rejection, neuroinflammation, neuroregeneration, and pain. She is the inventor of the first nanoparticle-based oxygen delivery agent tested on human organ/tissue preservation and the inventor of the first neuroinflammation-targeted nanomedicine analgesic. Her work in nanomedicine and biomaterials has been extensively supported by CDMRP, USAF, and NIH (NIBIB, NIDA). She shares her pragmatic approach to manufacturing in nanomedicine with her students, both in her research laboratory and by teaching manufacturing in the graduate program at Duquesne University for over a decade. She is passionate about solving manufacturing problems to expedite bringing high-quality nanomedicines to patients.
KinetiSol®: Revolutionizing Formulation and Sustainable Manufacturing for the Future
of Amorphous Solid Dispersions
Dave Miller, PhD
CSO, AustinPx
Abstract:
KinetiSol is a novel fusion-based process to produce amorphous solid dispersion systems that has been adapted for pharmaceutical processing from the plastics recycling industry. With its unique process attributes, KinetiSol is providing novel solutions for difficult to process poorly water-soluble compounds. These unique attributes include brief processing times, low temperatures, high mixing intensity, and high torque output. These aspects of the process offer unique capabilities for amorphous dispersion production with thermally sensitive pharmaceutical materials, high melting point APIs, and highly viscous polymers. Moreover, KinetiSol offers the operational, environmental, and economic benefits of non-solvent processing. The fundamentals of the KinetiSol process and its novel applications to the field of amorphous solid dispersion processing will be discussed during this presentation.
of Amorphous Solid Dispersions
Dave Miller, PhD
CSO, AustinPx
Abstract:
KinetiSol is a novel fusion-based process to produce amorphous solid dispersion systems that has been adapted for pharmaceutical processing from the plastics recycling industry. With its unique process attributes, KinetiSol is providing novel solutions for difficult to process poorly water-soluble compounds. These unique attributes include brief processing times, low temperatures, high mixing intensity, and high torque output. These aspects of the process offer unique capabilities for amorphous dispersion production with thermally sensitive pharmaceutical materials, high melting point APIs, and highly viscous polymers. Moreover, KinetiSol offers the operational, environmental, and economic benefits of non-solvent processing. The fundamentals of the KinetiSol process and its novel applications to the field of amorphous solid dispersion processing will be discussed during this presentation.
|
Bio: Dave A. Miller, Ph.D. is the Chief Scientific Officer at AustinPx. Prior to his current position, Dr. Miller served as Vice President of Research and Development at DisperSol Technologies for ten years. Before joining DisperSol, he was a Senior Principal Scientist at Hoffmann-La Roche. Dr. Miller specializes in formulation and processing technologies for improving oral bioavailability of insoluble small molecules. He has applied his expertise toward advancing numerous drug candidates through all stages of development from early discovery to line extension. Dr. Miller is an original inventor of the pharmaceutical applications of the KinetiSol technology and continues to be a primary innovative driver for application and expansion of the drug delivery platform. |
He has co-authored 43 research articles in peer-reviewed journals, co-authored 8 book chapters, and is co-editor of the First, Second, and Third Editions of the textbook, Formulating Poorly Water-Soluble Drugs. He is a co-inventor on 11 granted US patents and numerous granted and pending patents worldwide. Dr. Miller holds a B.S. in Chemical Engineering and a Ph.D. in Pharmaceutics from the University of Texas at Austin.
Innovative MCC Morphology to Reduce Tableting Challenges
Raxit Mehta
Technical Director, Asahi Kasei America, Inc
Abstract:
Microcrystalline cellulose (MCC) is a the most commonly used binder in the pharmaceutical formulations. Compressibility and flow property of MCC can be optimized and improved with help of particle morphology. In this presentation, we will discuss how unique morphology of high-performance MCC can improve flow property, form strong tablets, and overcome common tableting issues. Moreover, low nitrite impurity in the Ceolus MCC (0.1PPM or less) minimize the risk of nitrosamine formation in the drug products.
Bio:
Raxit Mehta has 17+ years of experience in developing oral solids dosage forms, from taste masked ODT to modified release tablets and multiarticulates. He enjoys interacting with the scientists to address formulation and tableting challenges. He has authored several papers, presented his work at international conferences, and served as a lead of excipient community at AAPS. He holds master’s degree in chemical engineering from The City College of New York and a bachelor’s degree in chemical engineering from Gujarat University, India
Raxit Mehta
Technical Director, Asahi Kasei America, Inc
Abstract:
Microcrystalline cellulose (MCC) is a the most commonly used binder in the pharmaceutical formulations. Compressibility and flow property of MCC can be optimized and improved with help of particle morphology. In this presentation, we will discuss how unique morphology of high-performance MCC can improve flow property, form strong tablets, and overcome common tableting issues. Moreover, low nitrite impurity in the Ceolus MCC (0.1PPM or less) minimize the risk of nitrosamine formation in the drug products.
Bio:
Raxit Mehta has 17+ years of experience in developing oral solids dosage forms, from taste masked ODT to modified release tablets and multiarticulates. He enjoys interacting with the scientists to address formulation and tableting challenges. He has authored several papers, presented his work at international conferences, and served as a lead of excipient community at AAPS. He holds master’s degree in chemical engineering from The City College of New York and a bachelor’s degree in chemical engineering from Gujarat University, India
Vendor presentation
Flux assay results: What do they mean and how can they help?
Abstract:
Dissolution-permeation or flux apparatus are commonly used in formulation development to assess the complex impact of excipients on dissolution, solubility, and permeability in one assay setup. The resulting flux curves are utilized to rank-order formulations and to understand the effect of various excipients, however, it is often difficult to predict the true in vivo absorption difference these formulations will have based on these flux results only. This presentation aims to demonstrate a mechanistic modeling approach that utilizes flux curves as the primary input parameter and considers numerous physiological properties to provide in vivo absorption rate predictions. These data can then be used to support formulators in making more informed decisions on formulation strategy.
The presentation will introduce the general concept and use of flux devices through various case studies, then will discuss the modeling approach and its capabilities using marketed drug product examples, and will provide guidance to formulators on the assessment of the rate-limiting steps of absorption.
About Pion:
Pion offers unique instruments and services designed to empower scientists in making informed decisions throughout the entire lifecycle of food and drug formulation, spanning from Research and Development (R&D) to Manufacturing. Our commitment lies in wholistically assisting users to gain a comprehensive understanding of critical properties such as dissolution, solubility, permeation, ionization, and absorption of their compounds and drug products.
Additionally, Pion provides consistent user support in the areas of material production, application consultation, and instrument training. We stand as an expert in analytical services surrounding the characterization of in vitro drug performance and chemical characterization, and our in-house manufacturing pipeline allows for stable and supportive partnerships between us and our customers.
Abstract:
Dissolution-permeation or flux apparatus are commonly used in formulation development to assess the complex impact of excipients on dissolution, solubility, and permeability in one assay setup. The resulting flux curves are utilized to rank-order formulations and to understand the effect of various excipients, however, it is often difficult to predict the true in vivo absorption difference these formulations will have based on these flux results only. This presentation aims to demonstrate a mechanistic modeling approach that utilizes flux curves as the primary input parameter and considers numerous physiological properties to provide in vivo absorption rate predictions. These data can then be used to support formulators in making more informed decisions on formulation strategy.
The presentation will introduce the general concept and use of flux devices through various case studies, then will discuss the modeling approach and its capabilities using marketed drug product examples, and will provide guidance to formulators on the assessment of the rate-limiting steps of absorption.
About Pion:
Pion offers unique instruments and services designed to empower scientists in making informed decisions throughout the entire lifecycle of food and drug formulation, spanning from Research and Development (R&D) to Manufacturing. Our commitment lies in wholistically assisting users to gain a comprehensive understanding of critical properties such as dissolution, solubility, permeation, ionization, and absorption of their compounds and drug products.
Additionally, Pion provides consistent user support in the areas of material production, application consultation, and instrument training. We stand as an expert in analytical services surrounding the characterization of in vitro drug performance and chemical characterization, and our in-house manufacturing pipeline allows for stable and supportive partnerships between us and our customers.
How AI, Machine Learning & Modeling Accelerate Discovery and Beyond
Abstract:
The world is excited about the potential impact of artificial intelligence (AI), machine learning (ML), and complex modeling—but what can they actually do today?
In this presentation, Dan O’Connor, Sr. Director of Business Development at Simulations Plus, will showcase how AI and ML are integrated into commercially available software platforms and already helping companies with drug discovery and development. Attendees will hear how industry peers are using these technologies across disciplines and throughout the drug development lifecycle for cost savings and decreased time to market.
Attendees will also get a sneak peek at the next generation of PBPK prediction software, GastroPlus® X.
About Simulations Plus:
Serving clients worldwide for more than 25 years, Simulations Plus is a leading provider in the biosimulation market providing software and consulting services supporting drug discovery, development, research, and regulatory submissions. Simulations Plus offers solutions that bridge artificial intelligence (AI)/machine learning, physiologically based pharmacokinetics, quantitative systems pharmacology/toxicology(QSP/QST), and population PK/PD modeling approaches.
Simulations Plus offers the #1-ranked, easy-to-use software (GastroPlus®, ADMET Predictor®, DILIsym®, NAFLDsym®, MonolixSuite® and more) to bridge data mining and compound library screening with QSAR models, PK/PD model development, and PBPK/TK modeling & simulation in animals and humans following administration around the body, and quantitative systems pharmacology approaches.
Simulations Plus technology and consulting services are utilized by major pharmaceutical, biotechnology, and regulatory agencies worldwide. For more information, visit our website at https://www.simulations-plus.com/.
Abstract:
The world is excited about the potential impact of artificial intelligence (AI), machine learning (ML), and complex modeling—but what can they actually do today?
In this presentation, Dan O’Connor, Sr. Director of Business Development at Simulations Plus, will showcase how AI and ML are integrated into commercially available software platforms and already helping companies with drug discovery and development. Attendees will hear how industry peers are using these technologies across disciplines and throughout the drug development lifecycle for cost savings and decreased time to market.
Attendees will also get a sneak peek at the next generation of PBPK prediction software, GastroPlus® X.
About Simulations Plus:
Serving clients worldwide for more than 25 years, Simulations Plus is a leading provider in the biosimulation market providing software and consulting services supporting drug discovery, development, research, and regulatory submissions. Simulations Plus offers solutions that bridge artificial intelligence (AI)/machine learning, physiologically based pharmacokinetics, quantitative systems pharmacology/toxicology(QSP/QST), and population PK/PD modeling approaches.
Simulations Plus offers the #1-ranked, easy-to-use software (GastroPlus®, ADMET Predictor®, DILIsym®, NAFLDsym®, MonolixSuite® and more) to bridge data mining and compound library screening with QSAR models, PK/PD model development, and PBPK/TK modeling & simulation in animals and humans following administration around the body, and quantitative systems pharmacology approaches.
Simulations Plus technology and consulting services are utilized by major pharmaceutical, biotechnology, and regulatory agencies worldwide. For more information, visit our website at https://www.simulations-plus.com/.
The Evolution of Drug Dosage Forms and the Apparatus 4
Abstract:
The evolution of drug dosage forms has presented significant challenges to traditional release testing methodologies. Nevertheless, Apparatus 4 has proven adept at addressing these challenges, solidifying its status as a critical technology within the pharmaceutical industry. Leveraging SOTAX Pharma Services, a dissolution-focused Contract Research Organization (CRO), Apparatus 4 has been instrumental in developing dissolution methods for particularly complex dosage forms, including implants and nanoparticle drug suspensions.
The testing of implants, characterized by their polymer matrix and the requirement for extended run times at elevated temperatures, poses a significant challenge. Traditional compendial apparatuses are inadequate for developing dissolution methods that meet current standards. However, Apparatus 4 is capable of accommodating complex media options and sustaining long run times at higher temperatures, thereby facilitating compliance with established guidelines. Nanoparticle suspensions introduce another layer of complexity due to the small particle sizes, which complicate sample filtration during dissolution testing. Apparatus 4 addresses this issue effectively, capable of filtering down to 25 nm while maintaining a compendial setup.
The capability of Apparatus 4 to adhere to the regulatory standards enforced by global agencies, and to accurately test complex dosage forms, underscores its importance as a leading tool in pharmaceutical release testing. This highlights the device's unparalleled utility and adaptability in the face of evolving pharmaceutical testing requirements.
About SOTAX:
With more than 50 years of experience, the SOTAX Group provides high quality solutions for the pharmaceutical Industry. With the US headquarters in Westborough, MA, SOTAX leads the way in the development and manufacture of Pharmaceutical Testing equipment, as well as offering services. Using our expertise in dissolution sciences, SOTAX provides innovative hardware solutions with the AT Xtend™ bath configuration, as well as method development, method validation and release testing using our newly expanded GMP laboratory. SOTAX Pharma Services is now available to leverage our niche as a stable platform for dissolution scientists in the need of a performance test.
Abstract:
The evolution of drug dosage forms has presented significant challenges to traditional release testing methodologies. Nevertheless, Apparatus 4 has proven adept at addressing these challenges, solidifying its status as a critical technology within the pharmaceutical industry. Leveraging SOTAX Pharma Services, a dissolution-focused Contract Research Organization (CRO), Apparatus 4 has been instrumental in developing dissolution methods for particularly complex dosage forms, including implants and nanoparticle drug suspensions.
The testing of implants, characterized by their polymer matrix and the requirement for extended run times at elevated temperatures, poses a significant challenge. Traditional compendial apparatuses are inadequate for developing dissolution methods that meet current standards. However, Apparatus 4 is capable of accommodating complex media options and sustaining long run times at higher temperatures, thereby facilitating compliance with established guidelines. Nanoparticle suspensions introduce another layer of complexity due to the small particle sizes, which complicate sample filtration during dissolution testing. Apparatus 4 addresses this issue effectively, capable of filtering down to 25 nm while maintaining a compendial setup.
The capability of Apparatus 4 to adhere to the regulatory standards enforced by global agencies, and to accurately test complex dosage forms, underscores its importance as a leading tool in pharmaceutical release testing. This highlights the device's unparalleled utility and adaptability in the face of evolving pharmaceutical testing requirements.
About SOTAX:
With more than 50 years of experience, the SOTAX Group provides high quality solutions for the pharmaceutical Industry. With the US headquarters in Westborough, MA, SOTAX leads the way in the development and manufacture of Pharmaceutical Testing equipment, as well as offering services. Using our expertise in dissolution sciences, SOTAX provides innovative hardware solutions with the AT Xtend™ bath configuration, as well as method development, method validation and release testing using our newly expanded GMP laboratory. SOTAX Pharma Services is now available to leverage our niche as a stable platform for dissolution scientists in the need of a performance test.
List of volunteers
Anand Gupta, Chair GSK |
Ka Ning Yip, Vice-Chair & Roundtable Lead Relay Therapeutics, Inc. |
Snehal Shukla, Treasurer Pfizer, Inc. |
Vivek Gupta, Past-Chair St. John’s University |
Harsh S. Shah, Sponsorship Lead J-Star Research, Inc. |
Ryan Williams CUNY |
Gautam Chauhan and Koyel Sen, Website and ARA Co-Leads Cipla and BI respectively |
Jia He Amgen |
Jennifer Chu, Program FreeThink Technologies |
Sameera Sansare Regeneron Pharmaceuticals |
Dana Gates, Sponsorship Application Lead Pfizer, Inc |
Dimple Modi GSK |
Heather Frericks Schmidt, STP Lead Pfizer, Inc |
John Pirro Sword Bio |
Jayshil Bhatt and Jelena Janjic, Poster Co-Leads Amgen and Duquesne University respectively |
Ling Zhu University of Connecticut |
Suraj Fanse, Registration coordinator Merck |
David Kwajewski Selling Science |
Gigi Zhao Insmed |
Tanu Mehta GSK/ViiV |
Veeran Kadajji J-STAR Research/Porton |
Luke Burroughs University of Connecticut |
McKenzie Roy University of Connecticut |
Saurabh Bhorkade University of Connecticut |
Ashwin Abhang University of Connecticut |
Pawan Pandey University of Connecticut |